Acids and Bases
Key Notes :
Understanding Acids and Bases:
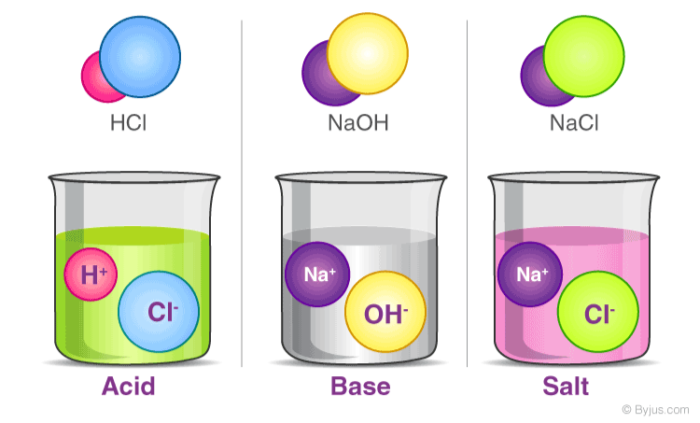
- Acids: Substances that release hydrogen ions (H⁺) when dissolved in water. They taste sour and can turn blue litmus paper red. Examples include lemon juice and vinegar.
- Bases: Substances that release hydroxide ions (OH⁻) when dissolved in water. They taste bitter and can turn red litmus paper blue. Examples include baking soda and soap.
Properties of Acids:
- Sour taste.
- Can conduct electricity in solution.
- React with metals to produce hydrogen gas.
- Examples: Citric acid in citrus fruits, acetic acid in vinegar.
Properties of Bases:
- Bitter taste.
- Slippery or soapy feel.
- Can conduct electricity in solution.
- Examples: Sodium hydroxide in drain cleaners, magnesium hydroxide in antacids.
pH Scale:
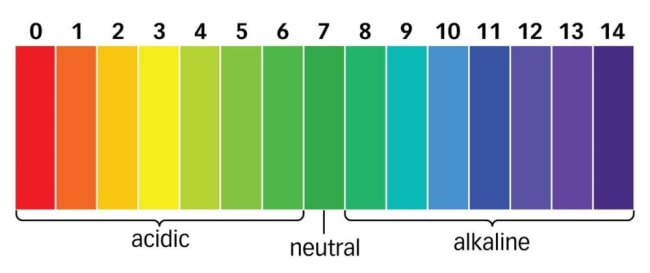
pH Scale: Measures how acidic or basic a substance is, ranging from 0 to 14.
- pH < 7: Acidic.
- pH = 7: Neutral (e.g., water).
- pH > 7: Basic (alkaline).
Indicators:
Litmus Paper: Changes color to indicate acidity or basicity.
- Red Litmus Paper: Turns blue in a base, stays red in an acid.
- Blue Litmus Paper: Turns red in an acid, stays blue in a base.
Universal Indicator: Changes color to show the pH level of a solution.
Neutralization Reaction:
Neutralization: A chemical reaction between an acid and a base that produces salt and water.
- Example: Hydrochloric acid (HCl) + Sodium hydroxide (NaOH) → Sodium chloride (NaCl) + Water (H₂O).
Safety:
- Always handle acids and bases with care.
- Wear safety goggles and gloves.
- Follow proper procedures for dilution and disposal.
Everyday Examples:
- Acids: Citrus fruits, soda, and yogurt.
- Bases: Toothpaste, baking soda, and ammonia.
Let’s practice!

