Neutralisation
Neutralization by Delta publications
Key Notes :
Definition of Neutralization:
- Neutralization is a chemical reaction between an acid and a base, resulting in the formation of water and a salt.
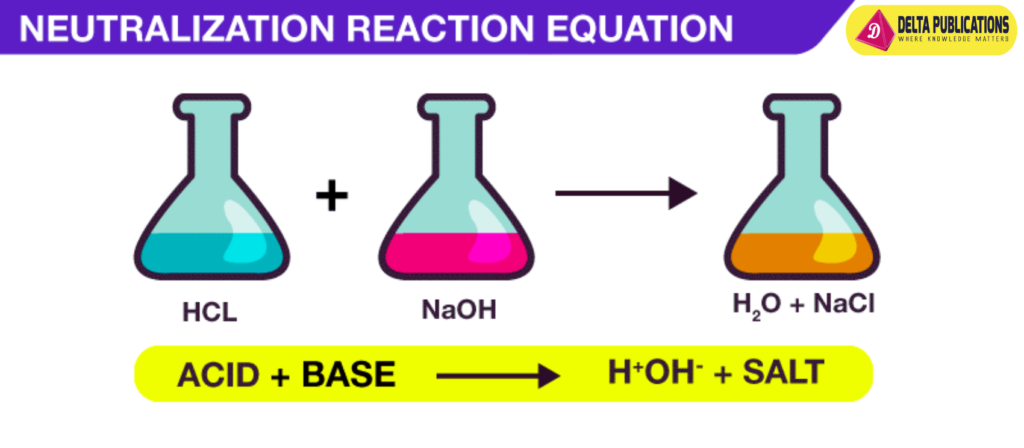
Basic Reaction:
- Acid + Base → Salt + Water
- Example: Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water (H₂O). HCl + NaOH → NaCl + H2O
pH Change:
- In neutralization, the pH of the solution moves closer to 7, which is neutral. The acidic or basic nature of the solution is reduced.
Importance of Neutralization:
- Neutralization reactions are important in everyday life, such as in antacids neutralizing stomach acid, or in agriculture where lime (a base) is added to acidic soil.
Exothermic Reaction:
- Neutralization reactions are usually exothermic, meaning they release heat.
Examples in Everyday Life:
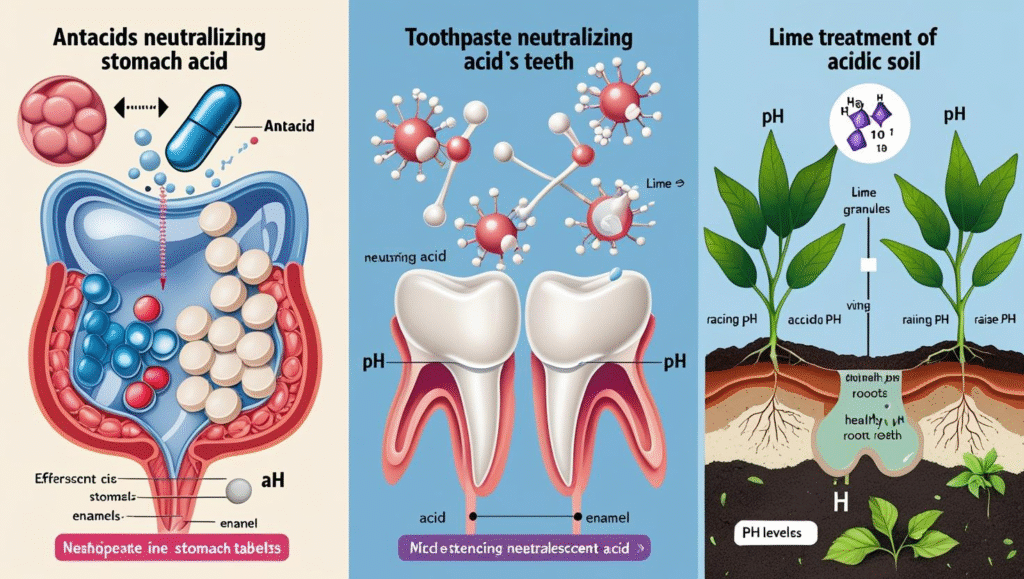
- Antacids: Neutralize excess stomach acid to relieve heartburn.
- Toothpaste: Neutralizes acids in the mouth to prevent tooth decay.
- Soil Treatment: Lime is used to neutralize acidic soils, improving plant growth.
Indicators in Neutralization:
- Indicators like litmus paper or phenolphthalein can be used to show the pH change during neutralization.

Applications in Industry:
- Neutralization is used in waste treatment processes to neutralize acidic or basic waste before discharge.
Balanced Equations:
- Understanding and writing balanced chemical equations for neutralization reactions is essential for understanding the process.
Let’s practice!

