Neutralisations In Everyday Life
Key Notes :
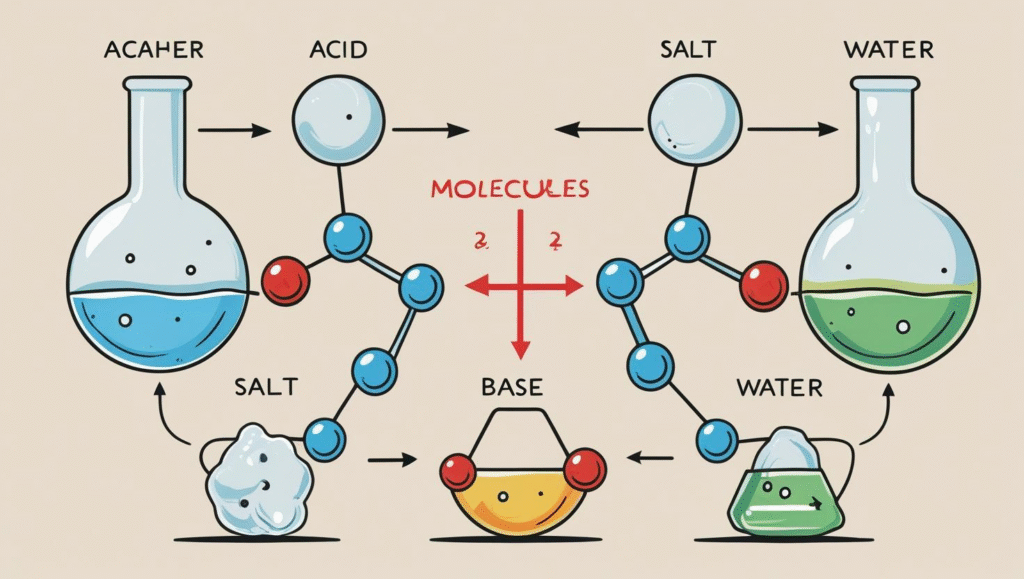
Definition of Neutralisation
- Neutralisation is a chemical reaction between an acid and a base to produce a salt and water.
- The general reaction is: Acid + Base → Salt + Water.
Importance of Neutralisation
- Neutralisation is important because it helps to balance pH levels in various environments, making substances safe and suitable for use.
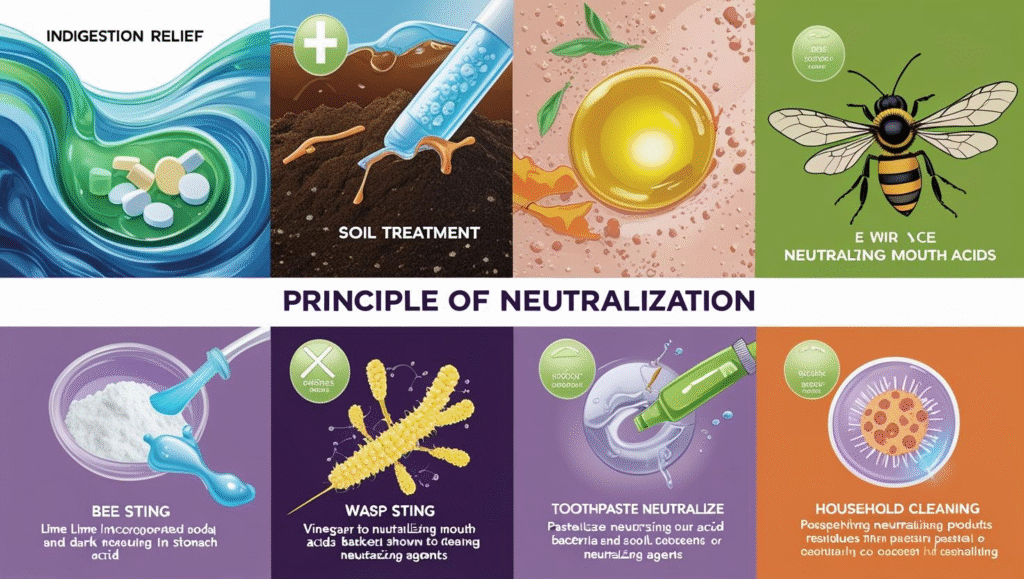
Everyday Examples of Neutralisation
- Indigestion Relief: Antacids are used to neutralize excess stomach acid, relieving heartburn.
- Soil Treatment: Lime (a base) is added to acidic soil to neutralize it, making it suitable for farming.
- Bee Stings: Baking soda (a base) can be applied to a bee sting (acidic) to neutralize the venom and reduce pain.
- Wasp Stings: Vinegar (an acid) can neutralize the alkaline sting of a wasp.
- Toothpaste: Contains mild bases to neutralize the acids produced by bacteria in the mouth, preventing tooth decay.
- Cleaning Products: Many household cleaning products are neutralizing agents that balance acidic or basic residues.
Environmental Neutralisation
Acid Rain: Limestone (a base) is used in lakes and soils to neutralize the effects of acid rain.

Industrial Waste: Neutralisation is used to treat acidic or basic waste products before they are released into the environment.

Neutralisation in Medicine
- Antacids: Medications that neutralize stomach acid to treat conditions like acid reflux and ulcers.
- Venom Treatment: Certain antivenoms work by neutralizing the effects of venom from bites or stings.
Neutralisation in Food
Cooking: Baking soda (a base) can neutralize the acidity of certain ingredients in recipes.
Preservation: Acids like vinegar are used in food preservation, and neutralization reactions can be used to balance flavors.
Indicators of Neutralisation
- pH Indicators: Substances like litmus paper can show when neutralisation has occurred by changing color.
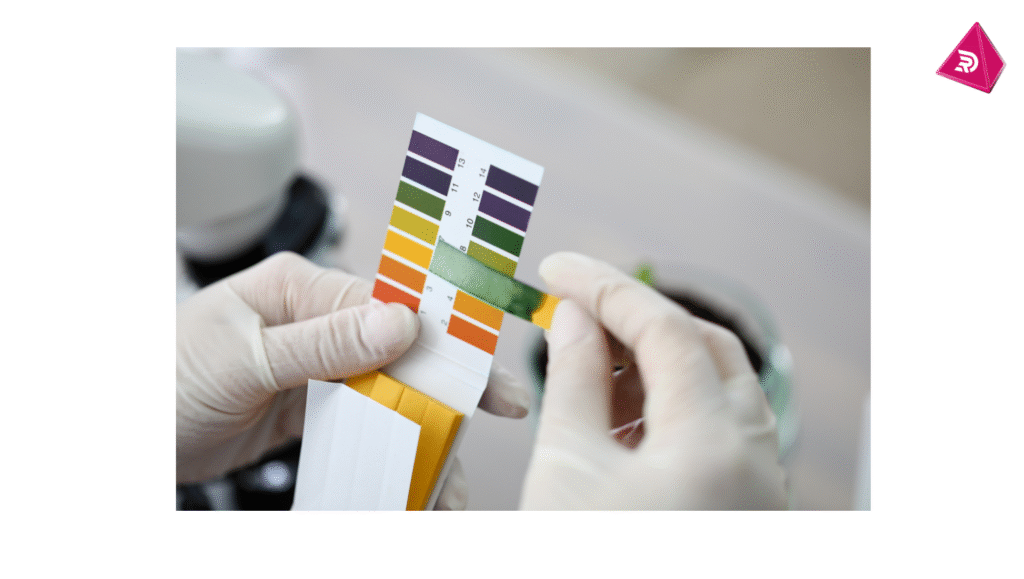
Balanced pH
- pH Scale: Neutralisation aims to bring the pH closer to 7, which is neutral on the pH scale.
- Safety: Ensuring a balanced pH is crucial for safety, comfort, and effectiveness in many everyday activities.
Let’s practice!

