Physical Changes
physical changes by Delta publications
Key Notes :
Definition of Physical Changes:
- Physical changes are changes in the form or appearance of a substance without altering its chemical composition.
- These changes are usually reversible, meaning the substance can return to its original state.
Characteristics of Physical Changes:
- No New Substance Formed: The substance undergoing the change remains the same at the molecular level.
- Reversibility: Most physical changes can be undone, such as melting ice into water and refreezing it.
- Energy Changes: Physical changes might involve energy changes, such as absorption or release of heat, but they do not involve the breaking or forming of chemical bonds.
- Change in State: A common physical change involves a change in the state of matter (solid, liquid, gas), like water freezing or evaporating.
Examples of Physical Changes:
- Melting: Ice melting into water.

- Freezing: Water freezing into ice.

- Boiling: Water boiling into steam.

- Condensation: Steam condensing into water.

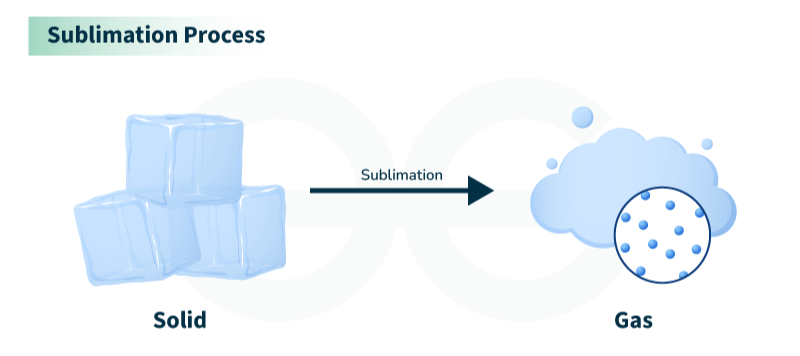
- Sublimation: Dry ice (solid carbon dioxide) sublimating into gas.
- Dissolving: Sugar dissolving in water.

Difference Between Physical and Chemical Changes:
- In physical changes, the substance’s physical properties change, but its chemical identity remains the same.
- In chemical changes, a new substance is formed with different chemical properties.
Importance of Physical Changes:
- Understanding physical changes helps in everyday life, like cooking, freezing food, or using substances like salt or sugar.
Observation of Physical Changes:
- Physical changes can often be observed through changes in shape, size, texture, or state of matter.
Real-life Applications:
- Physical changes are utilized in many processes, such as the purification of water through boiling and condensation (distillation) or the making of ice cream (freezing).
Let’s practice!

