Chemical Change
chemichal changes by Delta publications
Key Notes :
Definition of Chemical Change
- Chemical Change: A process that transforms one or more substances into new substances with different properties. It involves a chemical reaction and cannot be reversed by physical means.
Indicators of Chemical Change
- Color Change: A substance changes color (e.g., rust forming on iron).
- Temperature Change: Heat is absorbed or released (e.g., a chemical cold pack becoming cold).
- Formation of Gas: Bubbles or gas is produced (e.g., vinegar reacting with baking soda).
- Formation of Precipitate: A solid forms from a solution (e.g., mixing two clear solutions to form a cloudy solid).
Examples of Chemical Change
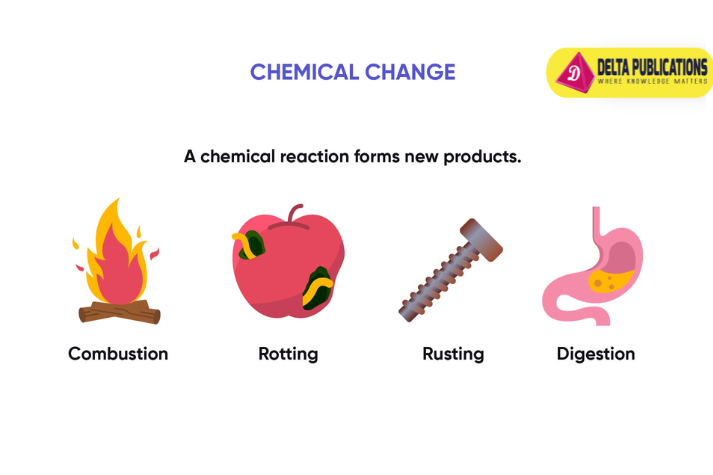
- Burning: Combustion of materials like wood or paper.
- Rusting: Iron reacts with oxygen and moisture to form rust.
- Cooking: Heating food changes its chemical composition (e.g., baking a cake).
- Digestion: The process of breaking down food in the stomach.
Chemical Reactions
- Reactants: Substances that undergo a chemical change.
- Products: New substances formed as a result of the reaction.
- Example: In the reaction of hydrogen and oxygen to form water, hydrogen and oxygen are reactants, and water is the product.
Conservation of Mass
- During a chemical change, the mass of the reactants equals the mass of the products. This principle is known as the Law of Conservation of Mass.
Reversibility
- Unlike physical changes, chemical changes are generally irreversible. For example, burning wood cannot be undone to return it to its original state.
Chemical Change vs. Physical Change
- Chemical Change: Results in new substances with different properties.
- Physical Change: Affects the form or appearance of a substance without changing its chemical composition (e.g., melting ice).
Safety Considerations
- Always handle chemicals with care and follow safety instructions during experiments to prevent accidents or injuries.
Let’s practice!

